Pediatric/Adult Airway Reconstruction/Regenerative Medicine
Collaborators:
Dr. Steven Goudy, Dr. April Landry, Dr. Kevin Maher, Dr. Subi Shashidharan, Dr. Dawn Simon, Emory University
Dr. Glenn Green, Dr. Rick Ohye, Dr. David Zopf, The University of Michigan
Airway Reconstruction: Tracheobronchal malacia is a condition in which the trachea and/or bronchi have reduced nonlinear elastic properties due to congenital or acquired conditions. These reduced properties can lead to partial or total collapse of the airway under exhalation. If severe, this condition can lead to respiratory arrest and death. We have developed computational models indicating that the severe form of this condition leads to respiratory arrest through mechanically induced airway instability. We have developed a patient specific, 3D printed resorbable splint that has been designated by the FDA under Humanitarian Use Device (HUD). We have implanted this device into 8 patients under 2 years old, allowing these patients to leave the ICU and come off mechanical ventilation. We are currently implementing a Quality System for this device so that it may be produced in our GMP 3D Printing facility for emergency use cases if needed (3DMedFab in the Georgia Institute of Technology Center for 3D Medical Fabrication).
In addition, we have developed a permanent form of the device that has been implanted to save the life of a 15 year old girl. We are developing a biologic form the device with the goal of generating cartilage rings external to the malacic airway for support, as well as a device for Laryngotracheal Reconstruction (LTR). Our long term goals are to develop regenerative medicine approaches to reconstruct cricroid cartilage and to engineer trachea and larynx.
Computational Modeling of Airway Instability and Collapse in Tracheomalacia

Example of tracheal instability and complete collapse due to malacia.
Splint Design, Modeling and 3D Printed Splint

Science and Translational Medicine 2015
Legend:
(A) Design parameters and image based design of resorbable airway splint
(B) Spiral splint model fit on digital model of patient airway
(C) Section of image based splint design created by custom MATLAB program
(D) Actual 3D Printed resorbable Polycaprolactone (PCL) splint shown operating room
Computational Simulation of Splint Performance
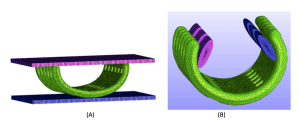
Nonlinear finite element simulation of designed splints
(A) Simulation of initial mechanical testing to determine geometric stiffness properties under compression
(B) Simulation of mechanical testing to determine splint opening stiffness to evaluate ability of splint to open during airway growth.
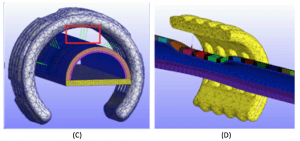
Nonlinear finite element modeling of malacic airway
(C) Model of splint on airway with nonlinear springs representing sutures (red box)
(D) Simulation (split view) showing splint maintaining airway patency
Splint Mechanism of Action and Patient Implantation of Splint
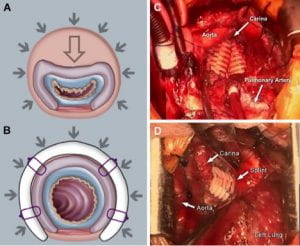
(A) Collapse of malacic airway
(B) Proposed splint mechanism to create patent airway
(C) Bilateral splint implantation in 2nd patient
(D) Splint implantation in 3rd patient
Splint in Patient at 6 months and 1 year
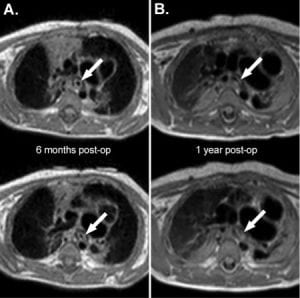
Science and Translational Medicine 2015
MRI of 1st patient with splint at 6 months (A) and 1 year (B). Arrows indicate dark ring around airway which is the implanted splint.
Splint Mechanism of Action and Patient Implantation of Splint

References:
Hollister SJ, Hollister MP, Hollister SK “Computational Modeling of Airway Instability and Collapse in Tracheomalacia”, (accepted), Respiratory Research.
Morrison RJ, Sengupta S, Flanagan CL, Ohye RG, Hollister SJ, Green GE, “Successful treatment of severe acquired tracheomalacia with a patient-specific 3D printed permanent tracheal splint”, (accepted), Otolaryngology – Head and Neck Surgery.
Hollister SJ, Flanagan CL, Morrison RJ, Patel JJ, Wheeler MB, Edwards SP, Green GE, “Integrating image-based design and 3D biomaterial printing to create patient specific device within a design control framework for clinical translation”, (2016), ACS Biomaterial Sci Eng, 2:1827-136.
Morrison RJ, Hollister SJ, Niedner MF, Park AH, Mehta DK, Ohye RG, Green GE “Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients”, (2015), Science Translational Medicine. , 7:285ra64.
Hollister SJ, Flanagan CL, Zopf DA, Morrison RJ, Nasser H, Patel JJ, Ebramzadeh E, Sangiorio SN, Wheeler MB, Green GE, “Design control for clinical translation of 3D printed modular scaffolds”, (2015), Annals Biomed Eng., 43:774-796
Morrison RJ, Kashlan KN, Flanagan CL, Wright JK, Green GE, Hollister SJ, Weatherwax KJ “Regulatory considerations in the design, manufacturing and clinical use of implantable 3D-printed medical devices”, (2015), Clinical & Translational Science, 8:594-600.
Zopf DA, Flanagan CL, Wheeler M, Hollister SJ, Green GE (2014) “Treatment of severe porcine tracheolmalacia with a 3-dimensionally printed, bioresorbable, external airway splint”, JAMA Otolaryngology: Head Neck Surg, 140:66-71.
Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE (2013) “Bioresorbable three-dimensional printed airway splint”, New England J. Medicine., 368:2043-2045.
Structural Heart Repair
Martin Bocks, Case Western Reserve University
Atrial septal defects(ASDs) are amongst the most commonly diagnosed congenital heart defects(CHDs). ASDs account for nearly 7-10% of all cardiac defects in children and 30-40% of all cardiac defects in adults. Untreated, ASDs can lead to pulmonary hypertension, systemic embolism, arrhythmias, and right sided heart failure. Current approved devices for transcatheter ASD secundum repair contain a Nitinol framework susceptible to metal erosion, failure and/or embolization. A bioresorbable transcatheter occluder that mechanically supports defect occlusion throughout endothelialization, tissue infiltration and defect reconstruction process can reduce the prevalence of long-term adverse events from metallic interatrial implants in an aging population.
Prototype PGD “Double Umbrella” Device

A prototype PGD “double umbrella” device framework is shown in 3 shape configurations: folded for delivery (A), with left atrial side deployed (B), and with both sides deployed (C). (Scale bar = 5 mm)
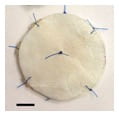
Top view of prototype occlusion device with attached SIS patches. (Scale bar = 5 mm)
Gross image of PGD device (star) following deployment within surgically created ASD (A). Left atrial side of device (star) after heart dissection (B).
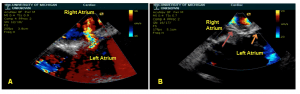
Intracardiac echocardiography (ICE) image of surgically created ASD with left to right shunt on color flow Doppler (A). ICE Image showing PGD occluder within the atrial septum and no residual atrial level shunting by color flow Doppler [Red arrow – Retention arm on right atrial side. Orange arrow – Retention arm on left atrial side] (B).
Craniofacial Soft Tissue Reconstruction
Work with Dr. David Zopf, Pediatric Otolaryngologist, Facial Plastics, University of Michigan; Dr. Matthew Wheeler, University of Illinois; Dr. Glenn Green, University of Michigan Pediatric Otolaryngology
Nasal and auricular reconstruction for adult patients following tumor resection or trauma and in pediatric patients especially due to congenital defects remains among the most challenging procedures in facial plastic surgery. This project combines image-based patient specific design with 3D biomaterial printing of polycaprolactone (PCL) to provide a scaffold reconstruction platform for the nose and ear. These scaffolds are readily combined with cells or growth factors to create a biologic construct to regenerate soft tissue in the correct patient specific anatomic shape. We are currently investigating optimal pore structure, cell type and mechanical property design to create the best reconstructive outcome.
Nasal Scaffold Image-based Boolean Design, 3D Printing and Implantation
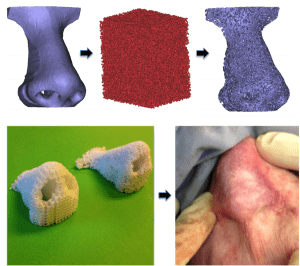
Otolaryngology – Head and Neck Surgery 2015
Top: Initial nasal anatomy (top left), image-based design random architecture (top middle), final image-based nasal scaffold design combining anatomy with designed architecture.
Bottom: 3D printed (laser sintered) polycaprolactone (PCL) nasal scaffolds with periodic (left) and random (right) pore architecture.
Auricular Scaffold Image-based Boolean Design, 3D Printing and Implantation

Otolaryngology – Head and Neck Surgery 2015
Image-Based Design: Auricular image-based 3D printed polycaprolactone scaffold, spherical pore design. Patient CT (upper left), STL rendering of individual auricle (upper middle), STL rendering with the addition of spherical porous microarchitecture using Boolean operation (upper right)
Fabrication: Laser sintered, 3D-printed polycaprolactone (PCL) auricular construct (lower left), 3D-printed PCL auricular construct with hyaluronic acid/type I collagen hydrogel containing 30 million chondrocytes embedded in PCL scaffold (lower middle), and after immediate porcine post-auricular subcutaneous implantation (lower right).
We are also determining the nonlinear elastic and viscoelastic properties of human and porcine (a commonly used model for craniofacial soft tissue reconstruction) nasal and auricular cartilage to use as design targets for the nasal and auricle reconstructive bioscaffolds.

Laryngoscope 2015

Laryngoscope 2015
Top: Mechanical testing of whole human ear in helix down testing
Bottom: Fit of Mooney-Rivlin nonlinear elastic model (solid red line) to experimental data (dotted blue line) from compression tests of human ear.
Reference for permission:
Zopf DA, Mitsak AG, Flanagan CL, Wheeler MB, Green GE, Hollister SJ (2015) “Computer-aided designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction”, Otolaryngology – Head and Neck Surgery., 152:57-62
Zopf DA, Flanagan CL, Nasser H, Mitsak A, Haq FS, Rajendran V, Green GE, Hollister SJ “Biomechanical evaluation of human and porcine auricular cartilage”, (2015) Laryngoscope., E262-268.
Craniofacial Hard Tissue Reconstruction
– Dr. Steven Goudy, Pediatric Otolaryngologist, Emory University; Dr. Shelly Abramowicz, Pediatric Oral/Maxillofacial Surgery, Emory University; Dr. William Giannobile, University of Michigan; Dr. Sean Edwards, Oral/Maxillofacial Surgery, University of Michigan; Dr. Matthew Wheeler, University of Illinois
Craniofacial, mandibular, and temporomandibular joint reconstruction due to tumor resection, trauma or congenital deformities is extremely challenging due to the geometric complexities of these structures, their load bearing requirements, and the bacterial environment of the mouth. This project combines image-based patient specific design, 3D printing and biologic (cells and growth factor) delivery for craniofacial bone and joint reconstruction. Image data is used for designing these complex structures, and in the case where a structure on one side is missing, symmetry is used for design by mirroring the unaffected structure. The resulting structures are then 3D printed along with associated fixation. Using this method, we have engineered mandibular condyle reconstruction in large animals (top panel), engineered a scaffold to deliver Platelet Derived Growth Factor (PDGF) for bone and soft tissue reconstruction in a patient with periodontal disease (middle panel) and engineered a pre-fabricated flap for large composite bone/soft tissue reconstruction in the mandible (bottom panel). The last application, pre-fabricated flaps, are meant to address the extremely challenging problem of vascularizing large composite tissue constructs and ensuring that they remain viable. A construct is designed to match the defect, matched with a biologic, and then implanted in a muscle bed for 1-2 months before being transplanted into the recipient site.
Mandibular Condyle Reconstruction using patient specific 3D Printed Scaffolds
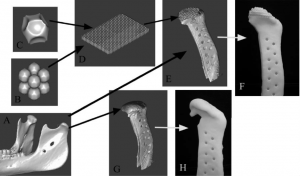
Int. J. Medical Robotics and Com. Assisted Surgery 2007
Example of image-based design procedure for mandibular condyle scaffold.
(A) CT scan of Yucatan minipig condyle used to design external anatomical shape and surgical fixation.
(B–D) Porous architecture databases based on geometrical equations for bone and cartilage region.
(E) Final integrated image design of mandibular condyle scaffold with external anatomical shape, surgical fixation, and interior designed porous architecture.
(F) Mandibular condyle scaffold architecture design fabricated from polycaprolactone, a degradable biopolymer.
(G) Final image design of mandibular condyle scaffold, demonstrating anatomical shape, surgical fixation and shell condylar region.
(H) Mandibular condyle scaffold shell design fabricated from polycaprolactone.
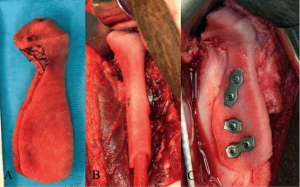
Int. J. Medical Robotics and Com. Assisted Surgery 2007
3D printed mandibular condyle replacement scaffold.
(A) Scaffold filled with marrow for reconstruction.
(B) Placement of scaffold on ramus using collar for fixation.
(C) Placement of screws to fix scaffold into placed on ramus
Bone/Soft Tissue Reconstruction in a Patient with Periodontal Disease using Scaffold Delivery of PDGF
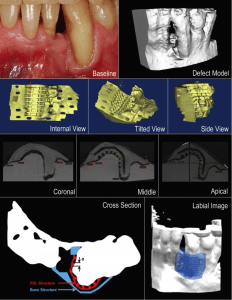
Dental Res. 2015
Design of biphasic periodontal repair scaffold.
Top: Initial patient condition and defect image.
Second Row: 3D view of scaffold design.
Third Row: Micro-CT image of 3D printed scaffold fit on 3D printed patient image model.
Last Row: Schematic of bone and periodontal ligament (PDL) regions within scaffold and digital model of scaffold fit into defect.

Dental Res. 2015
Scaffold implantation and clinical outcome.
Top Row: Baseline patient condition and preparation of scaffold recipient site.
Second Row: Sterilized scaffold prior to implantation; Scaffold implantation.
Third Row: Initial soft tissue closure over scaffold; Scaffold at site 2 months post implantation.
Fourth Row: Scaffold at site 6 months and 1 year post-implantation.
Patient Specific, 3D Printed Pre-fabricated Flap for Vascularized Large Craniofacial Defect Reconstruction

ACS Biomaterial Sci Eng 2016
Process of engineering a pre-fabricated free flap for large vascularized composite tissue reconstruction. An initial optimized design is created to match defect geometry with material distribution for load bearing. Design is 3D printed and biologics (in this case Bone Morphogenic Protein 2 – BMP2) are attached within the scaffold. Scaffold is implanted within a muscle bed. Scaffold is monitored within the muscle bed where bone (blue) grows within scaffold (yellow). Scaffold along with muscle and vascular pedicle is removed for transplant.
References:
Weisgberger DW, Erning K, Flanagan CL, Hollister SJ, Harley BAC “Evaluation of multi-scale mineralized collagen-polycaprolactone composites for bone tissue engineering”, (accepted), J. Mech. Behav. Biomed. Mat.
Pilipchuk SP, Monje A, Jiao Y, Hao J, Kruger L, Flanagan CL, Hollister SJ, Giannobile WV “Integration of 3D printed and micropatterned polycaprolactone scaffolds for guidance of oriented collagenous tissue formation in vivo”, (2016) Advanced Healthcare Materials. 5:676-687.
Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, Giannobile WV “3D printed bioresorbable scaffold for periodontal repair”, (2015), J. Dental Res., 94:153S-157S
Patel JJ, Modes JE, Flanagan CL, Krebsbach PH, Edwards SP, Hollister SJ “Dual delivery of EPO and BMP2 from a novel modular poly-e-caprolactone construct to increase bone formation in pre-fabricated flaps”, (2015), Tissue Eng C., 21:889-897.
Chanchareonsook N, Tideman H, Hollister SJ, Flanagan C, Jansen JA (2014) Mandibular reconstruction with a bioactive-coated cementless Ti6AL4V modular endoprosthesis in Macaca facicularis, Int. J Oral Maxillofac Surg., 43:758-768.
Park CH, Rios HF, Taut AD, Padial-Molina M, Flanagan CL, Pilipchuk SP, Hollister SJ, Giannobile WV (2014) “Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces”, Tissue Eng. C., 20:533-542
Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, Flangan CL, Hollister SJ, Giannobile WV (2012) “Tissue engineering bone-ligament complexes in vivo using fiber-guiding scaffolds”, Biomaterials, 33:137-145.
Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, Giannobile WV (2010) “Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces”, Biomaterials, 31:5945-5952.
Smith, M, Flanagan, CL, Kemppainen JM, Sack J, Chung H, Das S, Hollister SJ, and Feinberg SE. (2007) “Computed tomography-based tissue engineered scaffolds in craniomaxillofacial surgery”, Int. J. Medical Robotics and Com. Assisted Surgery, 3:207-216.
Mao, J.J., Giannobile, W.V., Helms, J.A., Hollister, S.J., Krebsbach, P.H., Longaker, M.T., and Shi, S., (2006) “Craniofacial tissue engineering”, J. Dental Research, 85:966-979.
Schek, R.M., Taboas, J.M., Hollister, S.J., and Krebsbach, P.H., (2005) “Tissue engineering osteochondral implants for Temporomandibular joint repair”, Orthodontics and Craniofacial Research. 8:313-319.
Feinberg, S.E, Hollister, S.J., Halloran, J.W., Chu, T.M., and Krebsbach, P.H.: Image-based biomimetic approach to reconstruction of the temporomandibular joint, Cells,Tissues, Organs, 169:309-321, 2001.
Hollister, S.J., Levy, R.A., Chu, T.M.G., Halloran, J.W., and Feinberg, S.E.: An Image Based Approach to Design and Manufacture Craniofacial Scaffolds, Int J of Oral/Maxillofacial Surgery, 29:67-71, 2000.


